Junior Research Group | FAIRRMeDIC
Background
Real-world evidence (RWE) is generated from studies using non-randomized data, especially real-world data (RWD) routinely collected in the healthcare system, such as in medical data integration centers (MeDICs). Although the potential of RWE and RWD for clinical practice is recognized, it is difficult to determine whether they can provide causal conclusions about treatment effects due to varying methodological rigor of corresponding studies. In this context, a recent article in the American medical journal JAMA calls for quality testing and assessment of reliability, relevance, and missing data, as well as adaptation of algorithms to increase validity1. Researchers and reviewers should be able to systematically assess whether RWD are appropriate for use by performing verification tests to assess reliability.
Real-world data is particularly sensitive and requires intensive protection, which must be a top priority, especially in MeDICs. Change control processes, such as those used in clinical studies or pharmacology, can make a decisive contribution here. Protection against re-identification must be prioritized in data sharing. This can be seen very clearly in a study on the risk of re-identification of data on Covid-19 cases that were released by the Portuguese government for research purposes2.
Aims
The Junior Research Groups focuses on the following goals:
-
Training of young scientists in the context of scientific method development and evaluation in a MeDIC
-
Expansion of the "FAIR" principles to include „Reliability“ with regard to MeDIC data and metadata
-
Quantification of the re-identification risk, e.g. in the context of applications for data sharing
-
Integration of extended change control processes for data security
-
Implementation of a multimodal user platform
The primary goal of the junior research group is to train young researchers. In order to achieve this, a scientific method development and evaluation for a medical data integration center (MeDIC) is carried out under the current aspects of the real implementation of a MeDIC.
A MeDIC in part holds and processes highly complex multidimensional data. Both the complexity and the volume of data are constantly increasing with new analysis and treatment methods in personalized medicine, e.g. molecular medicine. Methodological and technological concepts must therefore be adapted and further developed. This is the research focus of the FAIRRMeDIC junior research group. The FAIR principles "Findable, Accessible, Interoperable, Reusable" are to be strictly adhered and extended in the sense of "Reliable". A corresponding search of the literature revealed that only a few studies have dealt with reliable medical data or even considered an extension of the FAIR principles. In order to obtain reliable data and enable corresponding data analyses, the data must be validated. Above all, this means recording and scientifically assessing the quality of the data.
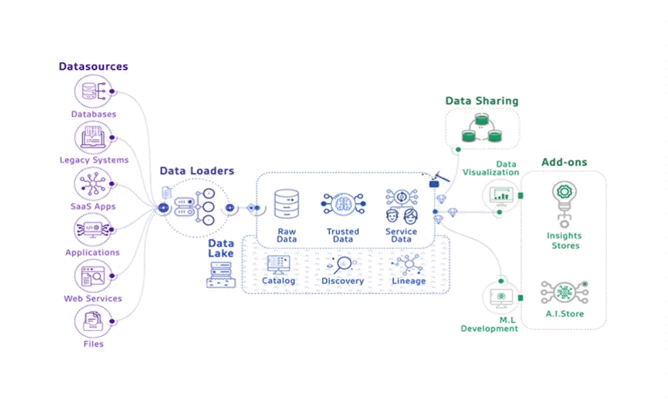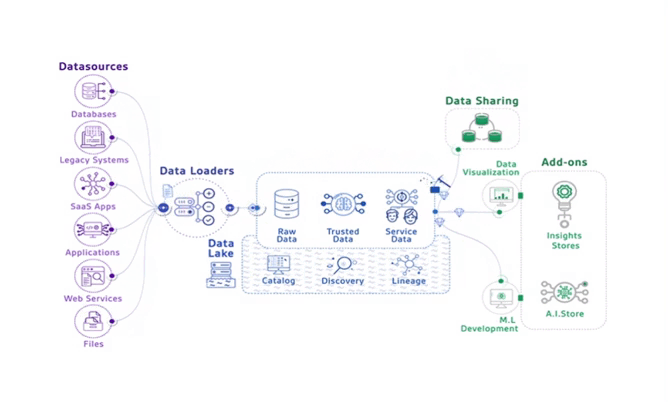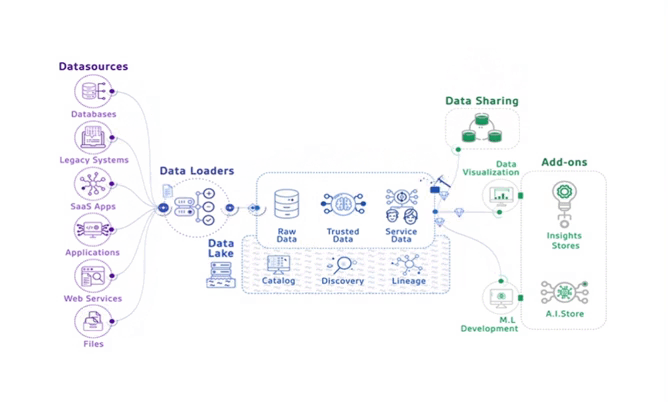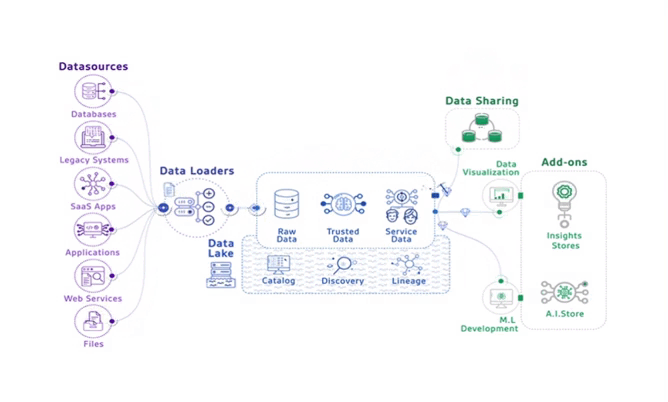
The MeDIC of the University Medical Center Göttingen (UMG) works strictly with pseudonymized data; data extraction and subsequent use of the data is regulated in principle. However, how
people can be re-identified when extensive medical data is integrated and analyzed has not yet been sufficiently investigated. To this end, the NWG is developing a procedure for stratifying the re-identification risk of medical data.
Figure: Projection of the MeDIC environment and services at the University Medical Center in Göttingen.
With the kind permission of Prof. Dr. Caroline Bönisch, team member of the NWG from 2022-2024.
Data must also be protected against unplanned modifications and changes must always be traceable. To achieve this goal, methods, principles and systems that are used in clinical research for the approval of drugs, such as the audit trail, are applied. Blockchain methods for securing data in the sense of a change control process have not yet been used on a large scale. The few studies on this come from the field of clinical trials or clinical pharmacology. For this reason, blockchain technologies are to be tested for application to medical data in a MeDIC.
Finally, a user-friendly system for analysis is to be established that meets the requirements of multidimensionality and quantity structures.
In summary, this means extending the FAIR principles in terms of reliability, investigating the intensive use of change control processes in a MeDIC and developing a method to quantify re-identification risk in order to provide researchers with high quality and secure data on a multimodal platform.
References
1. Wang SV, Schneeweiss S. Data Checks Before Registering Study Protocols for Health Care Database Analyses. JAMA. 2024, 331:1445-1446. doi: 10.1001/jama.2024.2988.
2. Carvalho T, Faria P, Antunes L, Moniz N. Fundamental privacy rights in a pandemic state. PLoS ONE. 2021 16: e0252169. https://doi.org/10.1371/journal.pone.0252169
Further Information on the MeDIC Göttingen
Head of the Junior Research Group FAIRRMeDIC
PD Dr. Dorothea Kesztyüs
University Medical Center Göttingen | Institute of Medical Informatics
Members of the Junior Research Group FAIRRMeDIC
Horim Bae
PhD Student
University Medical Center Göttingen


